Treatment of acid mine drainage (AMD)
TREATMENT OF ACID DRAINAGE OF MINES AIMING TO OBTAIN SULFURIC ACID AND METAL RECOVERY
Acid Mine Drainage (DAM) results from the natural oxidation of sulfide minerals that occurs when in the mining process they are exposed to air or water. DAM is characterized by being a highly acidic effluent and high concentrations of metal sulphate anions (mainly aluminum, copper, iron, magnesium, manganese and zinc sulphate) and organic compounds. Its formation reaction can be, simply, described by the oxidation of pyrite (FeS2):
2Fe2S + 7O2 + 2H2O 2Fe2SO4 + 2H2SO4
This effluent is considered as one of the most worrying environmental problems for the environmental inspection agencies and for the mining companies, as it causes irreversible consequences to the environment, such as: contamination of the soil and water bodies, erosion, silting of surface waters, in addition to of reducing the biodiversity of aquatic and terrestrial ecosystems. The present research aims to study the application of the electrodialysis (ED) technique in the treatment of this effluent in order to enable the reuse of water in the mining itself for the processing of coal. It is also expected to analyze the feasibility of obtaining sulfuric acid from this effluent as well as the concentration and recovery of metals.
The study will be developed using a bench ED cell with five compartments, as shown in Figures 1 and 2.
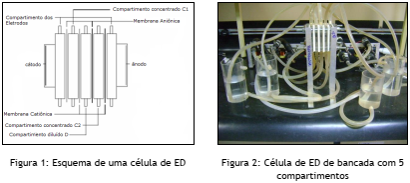
Between the compartments are placed alternating cationic and anionic membranes, with an area of 16cm² each. Cationic membranes prevent the passage of anions, while anionic membranes prevent the passage of cations. Examples of cationic and anionic membranes are shown in Figures 3 and 4.

Initially, in order to determine the limit current density of the process, polarization curves are obtained, obtained by applying increasing values of current densities between the anode and cathode at two minute intervals. After each interval, the potential values of the membranes are measured.
Once the limit current density is determined, ED will begin, where the passage of ionic species from the central diluted compartment to the concentrated cathodic or anodic compartments will be evaluated.
To determine the concentration of ions in the solutions, aliquots of the solution must be collected at pre-established time intervals. The results of transporting the ions through the membranes should be expressed in terms of percentage extraction and current efficiency.
After the ED test, the possibility of recycling metals and recovering sulfuric acid and water for reuse in mining will be evaluated, avoiding the disposal of wastewater.




 Imprimir
Imprimir